Home / 응용분야 / 상세페이지

면역 세포 치료
면역 치료를 위한 하나의 장비 시스템 ADAM-MC2, ADAM-CellT, ADAM-CDx
2017년 FDA는 혈액암(Blood cancer)에 탁월한 효능을 보인 CAR-T(키메라 항원 수용체 발현 T세포) 항암제를 허가했습니다. CAR-T에 대한 수요 증가와 이에 대응하는 연구 개발이 활발하게 진행되면서 정확한 면역 세포 수와 생존율이 주요 변수가 되었고. 높은 정확도와 재현성도 중요하게 되었습니다.
나노엔텍의 ADAM-MC2, ADAM-CellT, Neon*은 CAR-T 치료제의 연구개발, 생산, 품질관리 등에 활용될 수 있습니다. 또한, ADAMII-CDx**는 다양한 세포 작업과 분석을 하나의 장비로 수행할 수 있어 경쟁 장비 대비 합리적인 기기 가격과 검사 비용을 제공합니다. 전문가가 아니여도 간단한 교육만으로 사용 가능 하다는 장점이 있으며. 일회용 키트 사용으로 유지 보수에 부담이 없습니다.
*Neon 은 Thermo Fisher Scientific 상표입니다. 나노엔텍은 Neon이란 ODM 제품을 Thermo Fisher Scientific에 제공합니다.
**ADAMII-CDx는 나노엔텍에서 현재 개발중에 있는 제품입니다.
"면역 세포란 무엇인가?"
세포 치료제는 살아있는 세포를 이용해 손상된 세포를 회복하기 위한 재생을 유도하는 약물입니다. 신약개발, 독성검사, 바이오마커 등에 사용할 수 있습니다. 세포 치료제는 세 부분 조직 세포, 면역 세포, 줄기 세포 요법으로 나눌 수 있습니다. 최근 이슈되었던 면역 세포 치료법에 대해서 살펴보도록 하겠습니다.
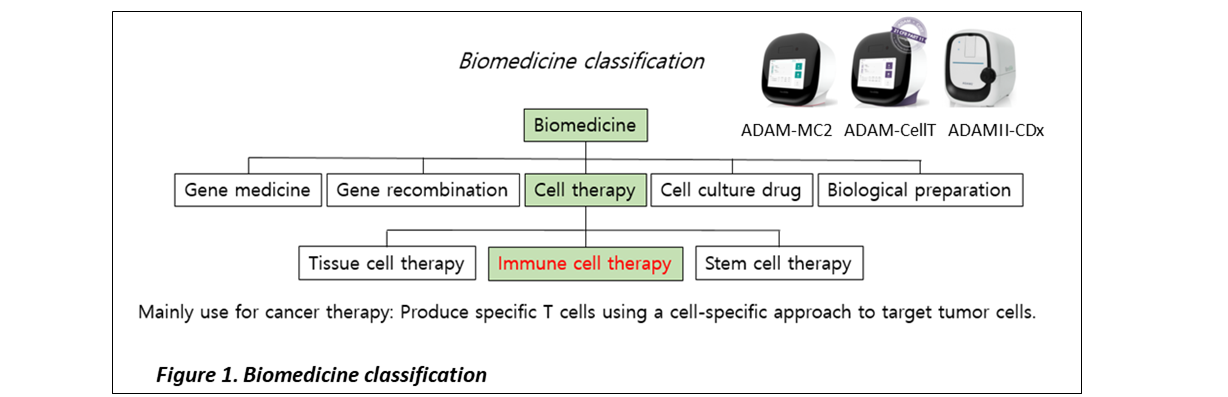
바이오약품은 세포 치료와 유전자 치료로 나눌 수 있으며, 세포 치료법은 조직 세포 치료, 면역 세포 치료, 줄기 세포 치료로 세분화 할 수 있습니다. 또한, 유전자 치료는 면역 세포 유전자 치료와 줄기 세포 유전자 치료로 구분될 수 있습니다. [1]
최근 혈액암에서 뛰어난 효율을 보여준 CAR-T세포를 이용한 면역 세포 치료에 대해서 자세히 알아보겠습니다.
CAR-T 세포의 정의와 적용
세포 치료제는 세포의 물질을 환자에게 주입시키는 것을 의미합니다. 이것은 살아있는 세포를 뜻합니다. 예를 들어, T세포는 면역치료를 통해서 투여된 면역으로 암세포와 싸워 치료가 가능합니다. CAR-T 세포는 인공의 T세포 수용기를 생산하는 역할을 해왔습니다. CAR-T세포 면역치료의 전제는 T세포를 변형시켜 암세포를 더욱 효과적으로 알아보고 망가트리기 위해서 입니다. 즉, 암 저항력의 중심인 면역세포를 채취하여 배양 및 활성화한 이후 다시 이식하여 암을 공격하는 치료법입니다. CAR-T세포는 환자의 자가 혈액에서 나오는 T세포로 부터 공급학나 다른 건강한 이의 혈액으로 부터 공급받습니다. 안전을 위해서, CAR-T세포는 건강한 세포가 아닌 종양에 있는 항원을 겨냥하도록 되어있습니다.
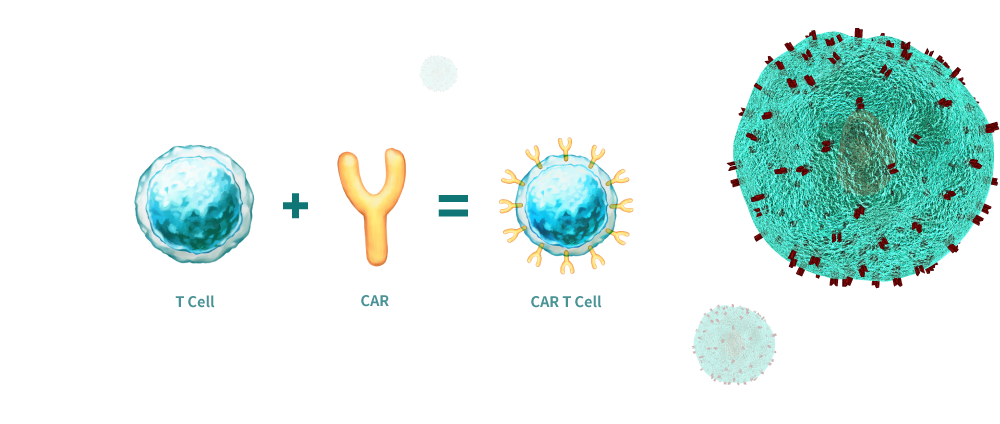
CD marker의 종류와 기능
면역 세포 요법은 체내의 면역 반응 활성화를 통해 질병을 치료하는 방법입니다. 즉. 암 저항력의 중심인 면역 세포를 채취하며 배양 및 활성화한 이후 다시 이식하여 암을 공격하는 치료법입니다. 면역세포 치료제 중 하나인 CAR-T(Chimeric antigen receptor T-cell)는 주입한 이후에 지속해서 증가하는 살아있는 본인의 T세포를 이용하여 종양 세포를 상상할 수 있는 특징을 지녔기에 '살아있는 약물'이라고 불립니다. [2]
일반적으로, 세포 표면 항원 무리 혹은 클러스터(CD,Cluster of differentiation)는 CD로 표기합니다 CD는 면역 표현형에 따라 세포 표면 분자를 식별하고 연구하기 위하여 붙여진 이름입니다. [3]
면역 세포의 치료제 이식의 성공을 위해서는 성숙 T-세포 (CD3), Helper T-세포(CD4), Killer T-세포 혹은 Suppressor T-세포(CD8) 등의 주요 분화 클러스터 (CD)의 양성 세포 수가 중요한 요인입니다.
왜 나노엔텍을 선택해야 하는가?
ADAM-MC 제품 시리즈 세포 치료적 응용
ADAM-MC 제품 시리즈가 세포치료제를 위해 세포를 배양하면서 세포의 개수와 생존율의 QC를 확인하는 장치로 씌이게 되었습니다. 게다가, ADAM-MC2 는 세포 종류 (Whole blood cell, PBMCs, etc)등에 따라 사용 가능합니다.
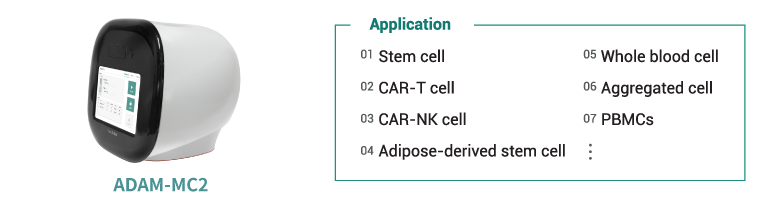

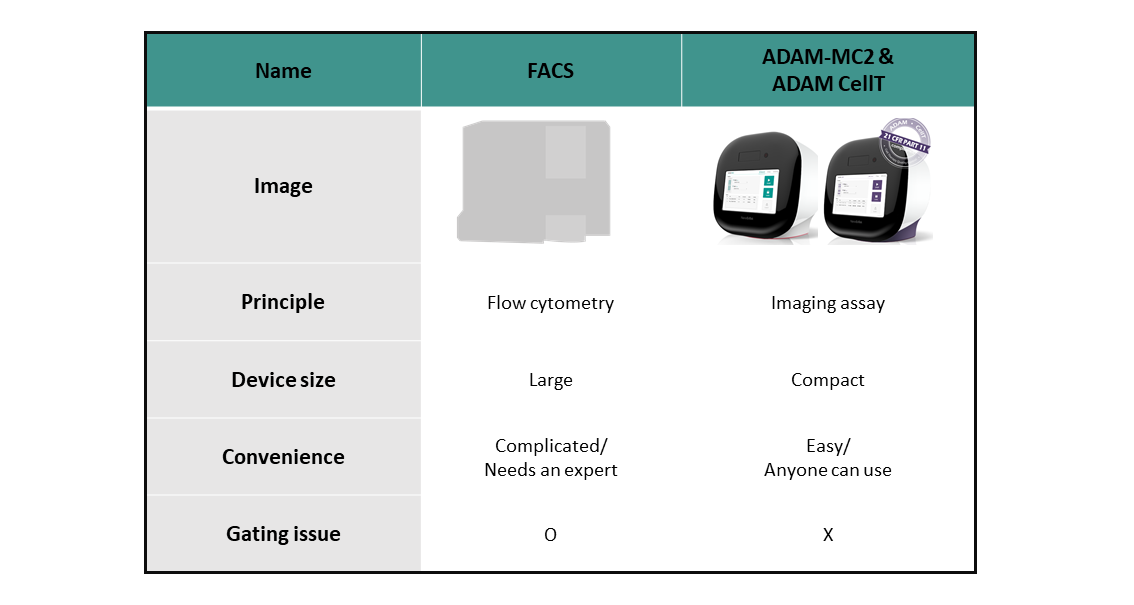
세포 치료제에 대한 연구와 생산의 수요가 세계적으로 증가하는 중에, 정확한 세포의 개수와 생존율 또한 중요한 요소가 되었습니다. 세포 개수를 세는 전통적인 방법인 유세포 분석기 (Flow cytometry)는 FACS (fluoresence-activated cell sorter)로 표현됩니다.[4]
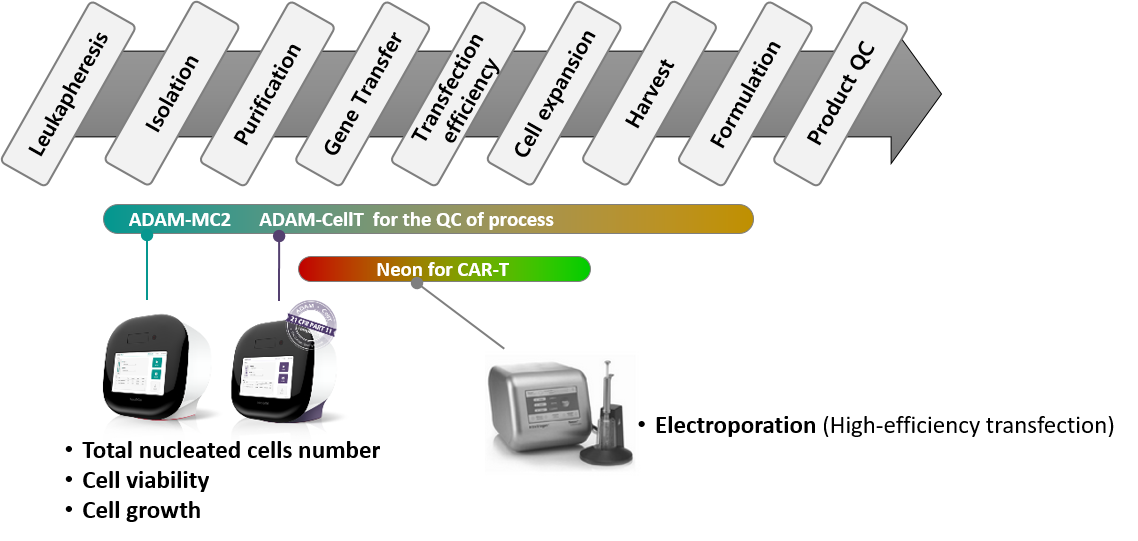
NanoEntek CAR-T's 플랫폼
CAR-T 제조 과정
새로운 혁신적인 제품, ADAMⅡ-CDx (곧 출시예정)
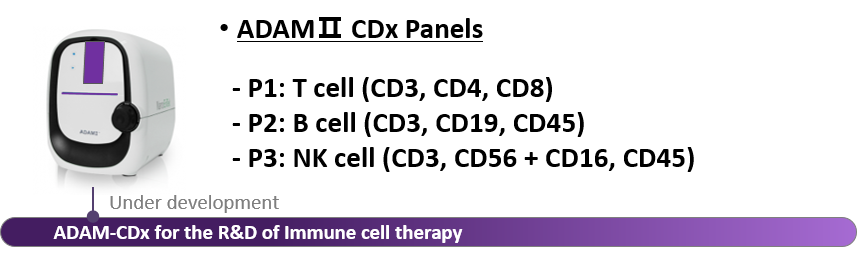
나노엔텍의 ADAMII-CDx은 현재 개발 진행중입니다. 이 장비는 백혈구 성분 분리 채집부터 Product QC까지의 세포 치료제 제조공정과 R&D에서 하나의 장비로 활용할 수 있는 기기입니다.
ADAMII-CDx는 T세포, B세포 그리고 NK세포등에 세포의 종류를 알아내고 세포의 개수, 크기 그리고 세포 주기를 파악하는데 특화되어 있습니다.
ADAM-MC2를 사용한 논문 자료
[3] Heterozygous frameshift variants in HNRNPA2B1 cause early-onset oculopharyngeal muscular dystrophy, Nature Communications, 13, 2306, 2022
참고 문헌
[1] Future of Cell Therapy in the Regenerative Medicine Market(2016), Frost&Sullivan, 나노엔텍 재가공
[2] Viardot, Andreas, et al. "Chimeric antigen receptor(CAR) T-cell therapy as a treatment option for patients with B-cell lymphomas: perspectives on the therapeutic potential of Axicabtagene ciloleucel." Cancer management and research 11(2019): 2393
[3] CHAN, J. K. C., NG, C. S., HUI, P. K.(1988). “A simple guide to the terminology and application of leucocyte monoclonal antibodies”. 《Histopathology》 12(5): 461–480.
[4] Huh, D.; WeiGu; Kamotani, Y.; Grotberg, J. B.; Takayama, S.Physiologicla Measurement 2005, 26, R73-R98.
[5] 채희정, and 윤덕현. "광범위큰 B 세포림프종에 대한 키메릭항원수용체 T-세포(Chimeric Antigen Receptor T-cells) 치료." Korean Journal of Medicine(구 대한내과학회지) 94.2(2019): 152-158.
- ADAM-CellT_Performance Evaluation of CAR-T cell KAI.pdf
- ADAM-MC2_Application note_Mammalian cell.pdf
- ADAM-MC2_ Polymorphic Region-Specific Antibody for Evaluation of Affinity-Associated Profile of Chimeric Antigen Receptor.pdf
- TDP-43 and PINK1 mediate CHCHD10S59L mutation_induced defects in Drosoph....pdf

 KOR
KOR ENG
ENG